Vancouver, BC – September 16, 2021 – Bold Therapeutics, a clinical-stage biopharmaceutical company developing BOLD-100, a first-in-class anti-resistance oncology therapeutic, has demonstrated potent anti-tumor activity in combination with a PD-1 checkpoint inhibitor in a validated I/O in vivo model of colorectal cancer. Cancer immunotherapies that blockade the PD-1/PD-L1 checkpoint, such as Merck’s Keytruda (pembrolizumab), BMS’ Opdivo (nivolumab) and more recently Jiansu Hengrui’s AiRuiKa (camrelizumab), have been shown to create durable therapeutic responses not typically seen with traditional anti-cancer therapies, improving patient outcomes in an increasingly wide range of indications and generating more than $23B in annual revenues worldwide. However, these therapies remain ineffective in a significant percentage of patients who are inherently resistant, and some patients who initially respond acquire resistance to these therapies over time. As the mechanisms underlying both inherent and acquired resistance to PD-1/PD-L1 are further elucidated, proactive anti-resistance therapeutic strategies could potentially result in both more frequent and improved patient outcomes.
In the experiment shown below, BOLD-100 was tested by an independent CRO in an MC-38 mouse model of colorectal cancer. BOLD-100 not only demonstrated potent monotherapy activity, as it has in other models, but also significantly improved outcomes when combined with a PD-1 inhibitor. Adding to an increasing wealth of literature, this data strongly supports BOLD-100’s potential to synergize with checkpoint inhibitors to reduce inherent and acquired resistance and improve patient outcomes. Further experiments are planned to explore and optimize BOLD-100’s utility in combination with checkpoint inhibitors in various established preclinical models with the goal of quickly advancing into a combination clinical study. BOLD-100 has already been shown to be generally safe and well-tolerated in a 46- patient monotherapy Phase 1 study, and Bold Therapeutics anticipates initiating numerous Phase 2 studies in 2022 and beyond.
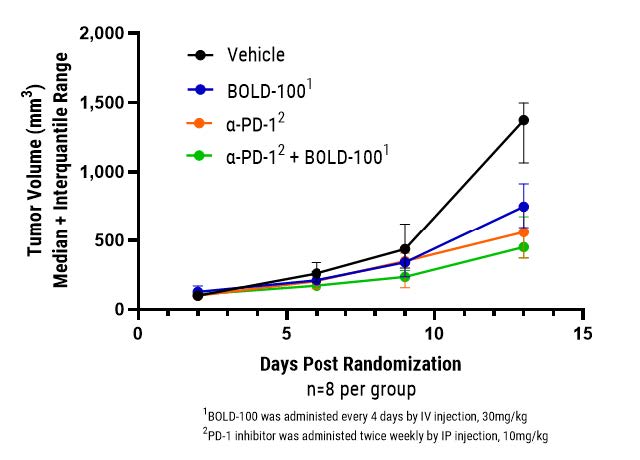
“These results in combination with a PD-1 checkpoint inhibitor adds to extensive preclinical and clinical evidence supporting BOLD-100’s broad utility,” stated E. Russell McAllister, CEO of Bold Therapeutics. “We have already demonstrated synergy between BOLD-100 and the therapies used in the majority of cancer care settings, ranging from traditional chemotherapies (e.g. cisplatin, gemcitabine) to targeted therapies (e.g. proteasome inhibitors, PARP inhibitors, receptor tyrosine kinase inhibitors – and now checkpoint inhibitors) to novel therapies (e.g. apoptosis and DNA damage pathway inhibitors). Drug resistance remains a significant unaddressed challenge in oncology, and Bold Therapeutics and our collaborators worldwide continue to demonstrate BOLD-100’s ability to address this unmet need by defeating both inherent and acquired resistance in a wide range of both solid and liquid tumor indications. Meanwhile, our ongoing seamless adaptive Phase 1b/2a clinical study is exploring the safety and efficacy of BOLD-100 in combination with FOLFOX in the treatment of advanced GI cancers – and we continue to expect to present results from the Phase 1b portion of the study at a conference in early 2022.”
For more information about Bold Therapeutics, please visit the company’s website at www.bold-therapeutics.com.
Source: Bold Therapeutics, Inc.
Contact:
E. Russell McAllister, CEO
