VANCOUVER, BC, Jan. 22, 2024 /PRNewswire/ — Bold Therapeutics, a clinical-stage biopharmaceutical company developing novel metallotherapeutics, presented positive Phase 2 safety and efficacy data in advanced metastatic colorectal cancer (mCRC) patients treated with BOLD-100 in combination with FOLFOX previously treated with FOLFOX/CAPOX at the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium on January 18-20, 2024.
Bold Therapeutics’ BOLD-100 is a first-in-class metallotherapeutic with a unique multimodal mechanism-of-action that targets a critical replication and survival pathway common across all cancers. Interim results presented at AACR (April 2023) and ASCO (June 2023) showed robustly positive efficacy and safety results in advanced colorectal, biliary tract and gastric cancer patients.
Bold Therapeutics’ ongoing BOLD-100-001 (NCT04421820) study is a multinational Phase 2 clinical trial evaluating BOLD-100 in combination with standard-of-care FOLFOX (folinic acid / leucovorin, fluorouracil, and oxaliplatin) in patients with advanced gastrointestinal cancers. The trial has currently enrolled 109 patients with advanced biliary tract, colorectal, gastric, and pancreatic cancers at sites in Canada, the United States, Ireland, and South Korea. The primary endpoint for the trial is progression-free survival (PFS), with overall survival (OS) and objective response rate (ORR) as secondary endpoints. Disease control rate (DCR) was also captured.
As of an August 31, 2023 data cutoff, data from 36 patients with advanced metastatic colorectal cancer was presented in clinical poster format (abstract #143) entitled “BOLD-100-001 (TRIO039): A Phase 2 Study of BOLD-100 in Combination with FOLFOX in Advanced mCRC Patients Previously Treated with FOLFOX/CAPOX: Efficacy and Safety Analysis.”
Key findings:
- Enrolled patients had a median of 4 [range 2-8] prior therapies including FOLFOX/CAPOX
- 67% of patients had progressive disease on prior FOLFOX/CAPOX
- Median progression-free survival (PFS) was 3.9 months [CI 2.7, 5.7]
- Median overall survival (OS) was 9.6 months [CI 6.0, 17]
- In 29 evaluable patients for response, the objective response rate (ORR) was 7.0%, with 2 partial responses (PR) and 20 stable diseases (SD) resulting in a disease control rate (DCR) of 76%
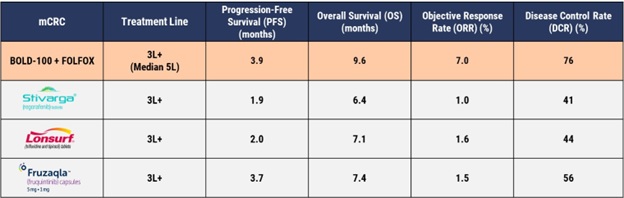
These findings indicate a clinical benefit for patients treated with BOLD-100 in combination with FOLFOX and on all metrics compares favorably to existing treatment options for advanced metastatic colorectal cancer: Taiho’s Lonsurf® (trifluridine / tipiracil), Bayer’s Stivarga® (regorafenib), and Takeda’s Fruzaqla® (fruquintinib). The table below compares this data against the data from the registration studies for these existing treatment options, all of which had less progressed (and thus healthier) patient populations.
BOLD-100 in combination with FOLFOX continued to be well-tolerated, with no new safety signals. For all treated patients, treatment-emergent adverse events (TEAEs) were observed in 33 (92%) of patients, with the most common TEAEs as follows: neutrophil count decreases (all grade 47%, grade ≥3 42%), nausea (42%, grade ≥3 0%), and fatigue (19%, grade ≥3 0%).
“Not only are we encouraged with BOLD-100’s efficacy, but we are excited about BOLD-100’s unexpectedly favorable safety profile, which has allowed patients to remain on treatment considerably longer than originally expected and thus maximizes the impact of the treatment combination,” noted Jim Pankovich, EVP of Clinical Development. “More specifically, relatively few patients in our study experienced neurotoxicity, despite all patients having been previously treated with neurotoxicity-inducing oxaliplatin.”
Phase 2 safety and efficacy results in biliary tract and gastric cancer are currently available under confidentiality and will be presented publicly at a major cancer conference later this year.
“Bold Therapeutics was founded in 2018 to improve patient outcomes in some of the most difficult-to-treat cancer indications and has rapidly progressed since then” added E. Russell McAllister, CEO. “We look forward to initiating late-stage studies in the near-future, initially targeting a 2027 FDA approval in advanced metastatic colorectal cancer.”
For more information, please visit the Company’s website at https://www.bold-therapeutics.com/
Contact: E. Russell McAllister, CEO
371735@email4pr.com
+1 (604) 262-9899
SOURCE Bold Therapeutics Inc.
