VANCOUVER, BC, April 18, 2023 /PRNewswire/ — Bold Therapeutics, a clinical-stage biopharmaceutical company developing first-in-class oncology therapeutics, announced positive interim results from a Phase 2 clinical trial evaluating BOLD-100 in combination with standard-of-care chemotherapy for the treatment of advanced colorectal cancer at the American Association for Cancer Research (AACR) 2023 conference.
Bold Therapeutics’ BOLD-100 is a first-in-class ruthenium-based small molecule therapeutic that (1) alters the unfolded protein response (UPR) through selective GRP78 inhibition; and (2) induces reactive oxygen species (ROS) which causes DNA damage and cell cycle arrest. Collectively, these effects result in cell death in both sensitive and resistant cancers, giving BOLD-100 the potential to significantly improve outcomes in a wide range of both solid and liquid tumors in combination with other anticancer therapies ranging from traditional chemotherapies to targeted therapies and immuno-oncology agents. Earlier preclinical data showed that BOLD-100 has potential immune modulating capabilities, including the induction of immunogenic cell death pathways. Preclinical data presented concurrently at AACR 2023 in a translational research poster (#2259) entitled “Novel Metallotherapeutic BOLD-100 Induces Circulating Cytokine Changes When Administered in Combination with FOLFOX in Advanced Gastrointestinal Cancer Patients,” show that BOLD-100 in combination with FOLFOX induced short-term plasma level changes in multiple cytokines, including IL-10 and IL-27, which help explain BOLD-100’s synergistic activity with a wide range of other anti-cancer drugs.
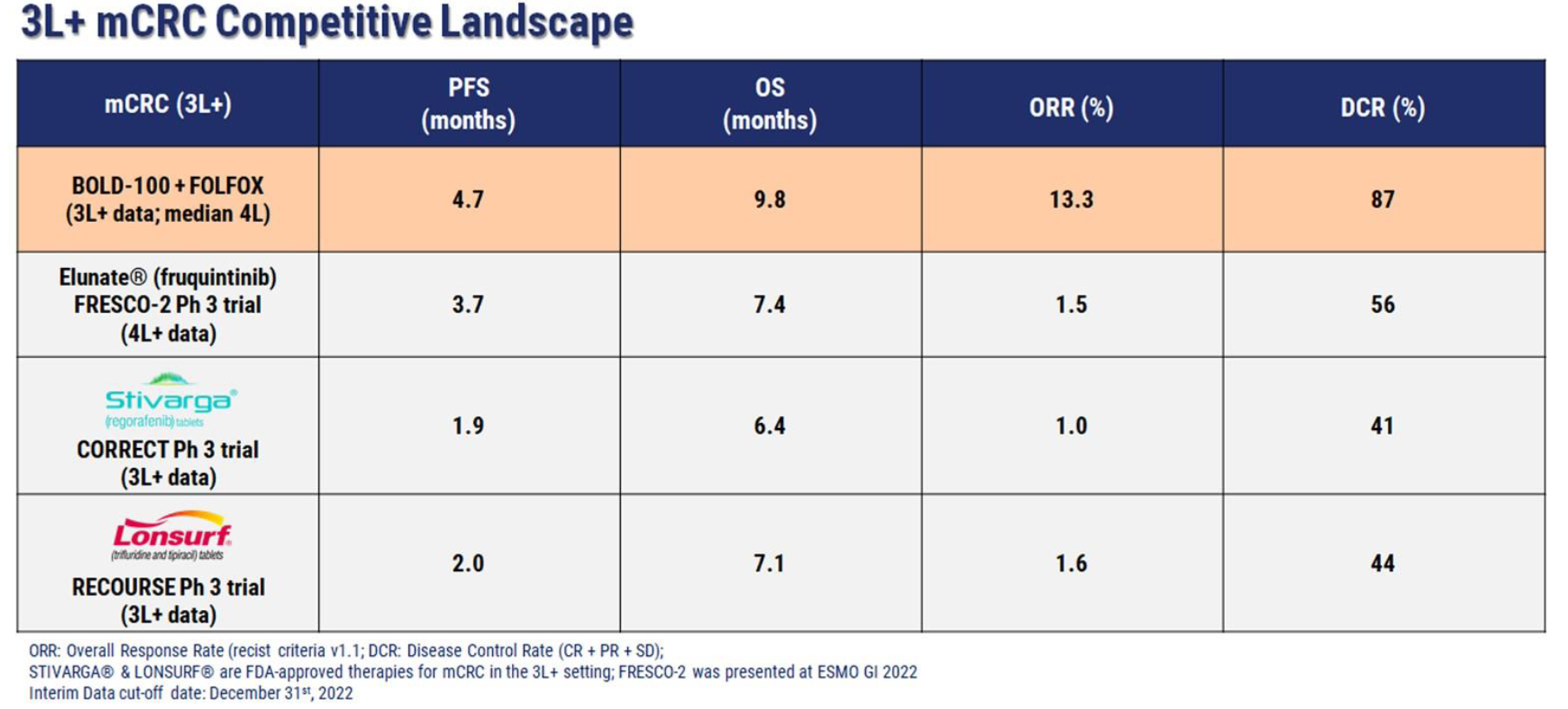
Bold Therapeutics’ ongoing multinational Phase 1b/2 trial (NCT04421820) evaluates BOLD-100 in combination with FOLFOX (folinic acid / leucovorin, fluorouracil, and oxaliplatin) in patients with advanced gastrointestinal cancers. The trial has currently enrolled 106 patients with advanced gastrointestinal (bile duct, colorectal, gastric, and pancreatic) cancers at sites in Canada, the United States, Ireland and South Korea, and the primary endpoint for the trial was progression-free survival (PFS), with overall survival (OS) and overall response rate (ORR) as secondary endpoints. Disease control rate (DCR) was also captured.
Data from 17 patients with advanced metastatic colorectal cancer was presented in clinical poster (#CT149) entitled “BOLD-100-001 (TRIO039): a Phase 1b/2a Dose-Escalation Study of BOLD-100 in Combination with FOLFOX Chemotherapy in Patients with Pre-treated Advanced Colorectal Cancer: Interim Efficacy, Safety and Tolerability Analysis.”
Patients were treated with BOLD-100 and FOLFOX, and the results from this difficult-to-treat patient group showed a median PFS of 4.7 months, OS of 9.8 months, ORR of 13%, and DCR of 87%, substantially higher than standard-of-care data (Bayer’s Stivarga® and Taiho’s Lonsurf®) for a similar patient population which showed a median PFS of up to 2.0 months, OS of up to 7.1 months, ORR of up to 1.6%, and DCR of up to 44%, with some of the most remarkable outcomes for BOLD-100 in late-line patients that had previously failed on FOLFOX alone. In addition, BOLD-100 in combination with FOLFOX proved to be exceptionally well-tolerated, with no new safety signals, with patients remaining on therapy for up to 15 treatment cycles.
“We are extremely pleased to announce this positive interim Phase 2 data at AACR and look forward to sharing even more clinical data in the coming months at ASCO. These results clearly demonstrate that BOLD-100 has the potential to significantly improve treatment outcomes in both solid and liquid tumors, beginning with advanced colorectal cancer, one of the most difficult-to-treat solid tumor indications” said E. Russell McAllister, CEO of Bold Therapeutics. “Perhaps most importantly, these results were achieved with a safety and tolerability profile that is almost indistinguishable from FOLFOX alone.”
Interim Phase 2 results in bile duct and gastric cancer will be presented at the upcoming American Society of Clinical Oncology (ASCO) conference in June, and data from the full Phase 2 trial, which will include an additional 20 patients with advanced colorectal cancer, should be available in late 2023. BOLD-100 was previously granted orphan drug designations (ODDs) in gastric and pancreatic cancer and expects additional ODDs and/or breakthrough therapy designations (BTDs) expected in 2023 and 2024. With a posterior probability of beating standard-of-care of 100% in PFS and 83% in OS in a head-to-head trial, Bold Therapeutics expects to initiate a pivotal Phase 3 trial for BOLD-100 in the treatment of advanced colorectal cancer in the near future and is currently evaluating potentially synergistic global development and commercialization partnerships. Concurrently, Bold Therapeutics is exploring additional development indications for BOLD-100 while also advancing its pipeline of other novel metallotherapeutics.
For more information, please visit Bold Therapeutics’ website at www.bold-therapeutics.com.
Additional information on AACR https://www.aacr.org/meeting/aacr-annual-meeting-2023/
Contact: E. Russell McAllister, CEO
(604) 262-9899
357453@email4pr.com
SOURCE Bold Therapeutics Inc.
